As humanity tries to reduce the global impact of greenhouse gases like carbon dioxide (CO2), we are encountering some fundamental problems.
First, while our energy demands are consistent, many low-carbon energy sources like solar panels only provide power intermittently. The excess power they generate can be used to create other fuels, like hydrogen gas, for use during low production periods, but we lack large storage facilities with the capacity to hold seasonal quantities of energy.
Second, mitigating the most severe effects of climate change will involve reducing the tremendous amounts of CO2 already in our atmosphere.
“The ultimate goal for these two gases is different,” said Assistant Professor Anna Herring. “For hydrogen, we want to be able to store it, then recover it efficiently. For CO2, we’re trying to find a safe and responsible way to dispose of it permanently.”
Fortunately, Herring believes that both problems can be resolved with one solution: underground gas storage.
Understanding Interactions Between States of Matter
Herring researches the complex multiphase interactions between gases, solids, and fluids, like saline water that occur in pockets of porous rock hundreds to thousands of meters underground. Such formations are common across the US, making them prime candidates for underground storage of hydrogen and CO2. However, the convoluted stone networks, high temperatures, extreme pressures, and unknown microbial interactions make successful gas storage hard to guarantee.
“It’s really important that we understand all the potential leakage pathways,” Herring said. “We need to constrain all of those different biological, chemical, and geological processes so that once we pump a gas underground, we know exactly where it is.”
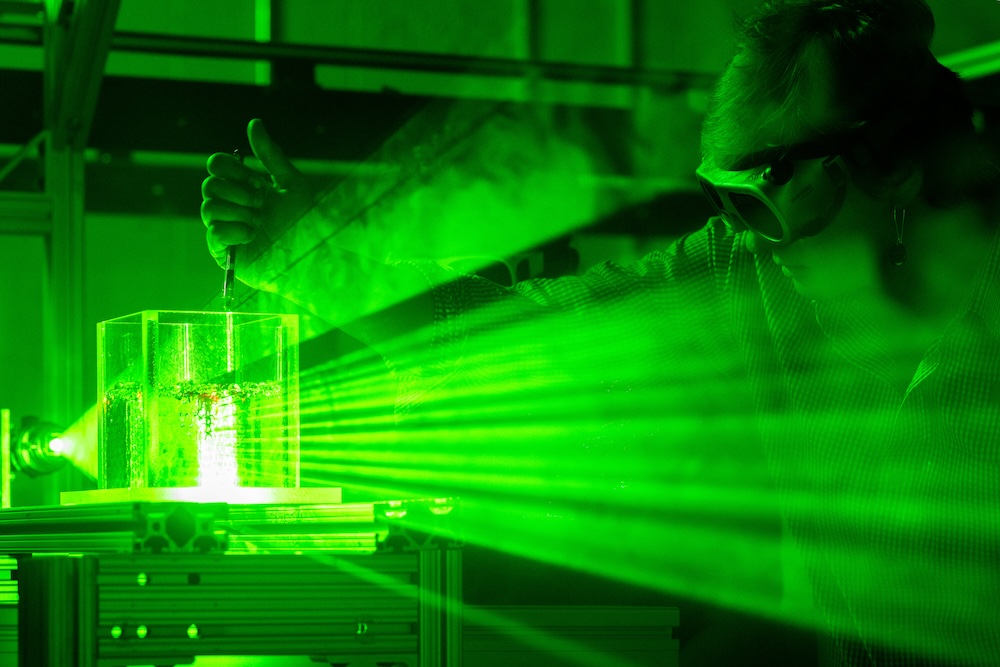
In her lab, Herring uses multiple experimental systems to understand the mass flow of gases in underground reservoirs. X-ray tomography and similar visualization tools help her observe the kinetics of gas bubble dissolution and movements through solid rocks. One of her PhD students, Christopher Allison, is studying how the surface chemistry of porous rocks changes when exposed to saline water and CO2 at the high pressures and variable temperatures found in subsurface environments.
Meanwhile, two undergraduate researchers, Sophia Hodges and Madeline Jenkins, are working to simulate how multiple immiscible fluids flow together through complex, porous rock systems.
“Hodges and Jenkins are learning the interesting fluid dynamics and physics relevant to the underground situations that we’re interested in,” Herring said. “Shadowing the PhD students also helps them understand how the simulations connect to the experimental work. We’re basically looking at the same process from two different perspectives.”
A New Perspective
Thanks to a recent $50,000 seed grant, Herring will soon be approaching the problem from yet another perspective. The funding, bestowed by UT’s Global Energy Ecosystems (GE2) strategic initiative and the Institute for a Secure and Sustainable Environment, will help Herring view multiphase reactions in unprecedented clarity—and safely bring hydrogen to the lab bench.
Joe-Sam Nkuah, another of Herring’s PhD students, has been conducting experiments with hydrogel beads—solid spheres with the same refractive index as water. A column filled with water and the hydrogel creates a porous architecture that is essentially invisible, allowing Nkuah and Herring to observe gas bubble dissolution on an entire plane using optical observation and lasers, rather than X-rays.
The hydrogel experiments are currently conducted with CO2 at room temperature and pressure, but Herring is hoping to integrate hydrogen soon.
“Hydrogen is flammable and explosive,” she said. “A large part of this seed grant is going towards establishing the safety protocols needed to safely run the experiments, including installing hydrogen sensors and safety shutoff valves.”
The Future of Herring’s Research
Once the safety protocols are in place, Herring plans to expand her work on underground hydrogen storage into a research group at UT. The group will be co-led by Assistant Professor Haochen Li and utilize the high-performance computing capabilities of his water infrastructure laboratory, the Psi Lab.
“Dr. Li’s expertise in computational and simulation studies complements my expertise in experimental design,” Herring said. “When you’re working with this kind of complex, interdisciplinary topic, it’s impossible for one person to understand everything. There’s a great collaborative network here at UT that we can leverage to really make sure that we’re looking at these processes comprehensively.”
Though underground hydrogen storage may still be a few decades from widespread implementation, Herring is excited to investigate its potential while simultaneously continuing to develop storage solutions for CO2.
“I think that these technologies are going to play a significant role in our country’s, and the world’s, attempts to mitigate climate change and transition to a renewable energy system,” she said. “This is a real opportunity to contribute to those goals, from the start, in a way that’s very impactful.”
Contact
Izzie Gall (865-974-7203, egall4@utk.edu)
